Lead Safe Mama, LLC’s CPSC Violation Report: LSM_04_2023: Pura Stainless knowingly sold Lead-contaminated children’s products through at least 2018. There was no recall. These products are still in use in homes today.
For those new to the Lead Safe Mama website:
Tamara Rubin is a multiple-federal-award-winning independent advocate for childhood Lead poisoning prevention and consumer goods safety, and a documentary filmmaker. She is also a mother of Lead-poisoned children (two of her four sons were acutely Lead-poisoned in 2005).
- Tamara owns and runs Lead Safe Mama, LLC — a unique community collaborative woman-owned small business for childhood Lead poisoning prevention and consumer goods safety.
- Since 2009, Tamara has been conducting XRF testing (a scientific testing method) using the exact instrumentation employed by the U.S. Consumer Product Safety Commission to test consumer goods for toxicants (specifically heavy metals — including Lead, Cadmium, Mercury, Antimony, and Arsenic).
- Since July of 2022, the work of Lead Safe Mama, LLC has been responsible for 5 product recalls (FDA and CPSC).
- All test results reported on this website are science-based, accurate, and replicable.
- Items that Lead Safe Mama, LLC reports on are tested multiple times to confirm the results published (for each component tested).
- Recent notable press… There has been too much to mention already in 2024! Please check out our press page to see some of the amazing coverage of our work so far this year!
This is an ad-free report.
Advertising and affiliate income help us cover the costs of the work (independent consumer goods testing and childhood Lead-poisoning prevention advocacy) we do here. We have published this report without advertisements to help make it easier to read. We have also removed ads from most of our more widely-read articles to make them easier for you to read. In addition to supporting this work by starting any shopping you might be doing with clicks on our affiliate links, if you would like to support our independent consumer goods testing and childhood Lead-poisoning prevention advocacy work by making a contribution (which will also help us keep our more widely-read articles ad-free), click here. Thank you!
Here’s a link to the PDF copy of this report as submitted to the CPSC.
Here’s a second link to the PDF version that was emailed to us as a confirmation.
A note to LeadSafeMama.com readers and community members
This note is NOT included in the violation report
If you have experienced this issue (the Lead-containing component of your child’s insulated Stainless Steel Pura Kiki insulated stainless steel baby bottle becoming exposed when the seal on the bottom of the product fails – for affected bottles which were available to be purchased through dates in 2018 and possibly available as late as dates in 2019 from third party vendors), please report the failure of your product (the exposure of Lead in the substrate caused by the bottom cap failing / falling off when the product is used as intended by a child) to the United States Consumer Product Safety Commission at the following link: https://www.saferproducts.gov/IncidentReporting.
- Please be prepared to include information about the design/ model and the date your item was purchased (as well as the vendor, if possible — Amazon, for example) as well as photos of the product that show the failure (if you have photos). If you have disposed of the item, photos are not required — please do file your report anyway (without photos).
- If your child has been tested for Lead (using Blood Lead Level [BLL] testing) please also include any positive test results that could be correlated to the child’s use of this product once the bottom cap failed and exposed Lead.
- In your report (if you have the following information) please include the date you purchased your product alongside the known (or approximate) date that the bottom cap fell off and use of the product began exposing your child to Lead. This will help to demonstrate how quickly these products failed in some cases (supporting that the use of these products presents a health risk to children).
Thank you.
Please feel free to use any of the language Lead Safe Mama, LLC has drafted (in our report below). The more reports that the CPSC receives about this violation of the Consumer Product Safety Improvement Act (CPSIA) of 2008, the more likely they will be to quickly take action in the matter.
Our goal in addressing this is for Pura Stainless to issue a highly visible public recall of their Lead-contaminated products (acknowledging their mistake and offering refunds and/or replacements to customers), and also that the CPSC issues a formal and official recall for any of the Lead-contaminated Pura Stainless products. In the report below we are also requesting that the CPSC investigate and determine (and publicize) the actual end date through which these Lead-contaminated baby bottles were manufactured as well as the actual end date through which these Lead-contaminated baby bottles were sold (directly from Pura Stainless or through other third party vendors).
Thank you for for being part of the Lead Safe Mama international advocacy community and thank you supporting this work in this way. Thank you for being part of the world-wide movement for consumer goods safety and childhood Lead poisoning prevention.
This is a VERY LONG report — as we needed it to be comprehensive and specific. We are accusing two entities of wrongdoing with this violation report, and it has been sent to a federal agency (the CPSC) for consideration. I don’t undertake these actions (or the responsibilities involved) lightly. Given the length of the piece below, I have chosen to remove all ads to make it easier to read. You can see more of the supporting documents and previous articles about these products (most of which are much shorter) by clicking this link.
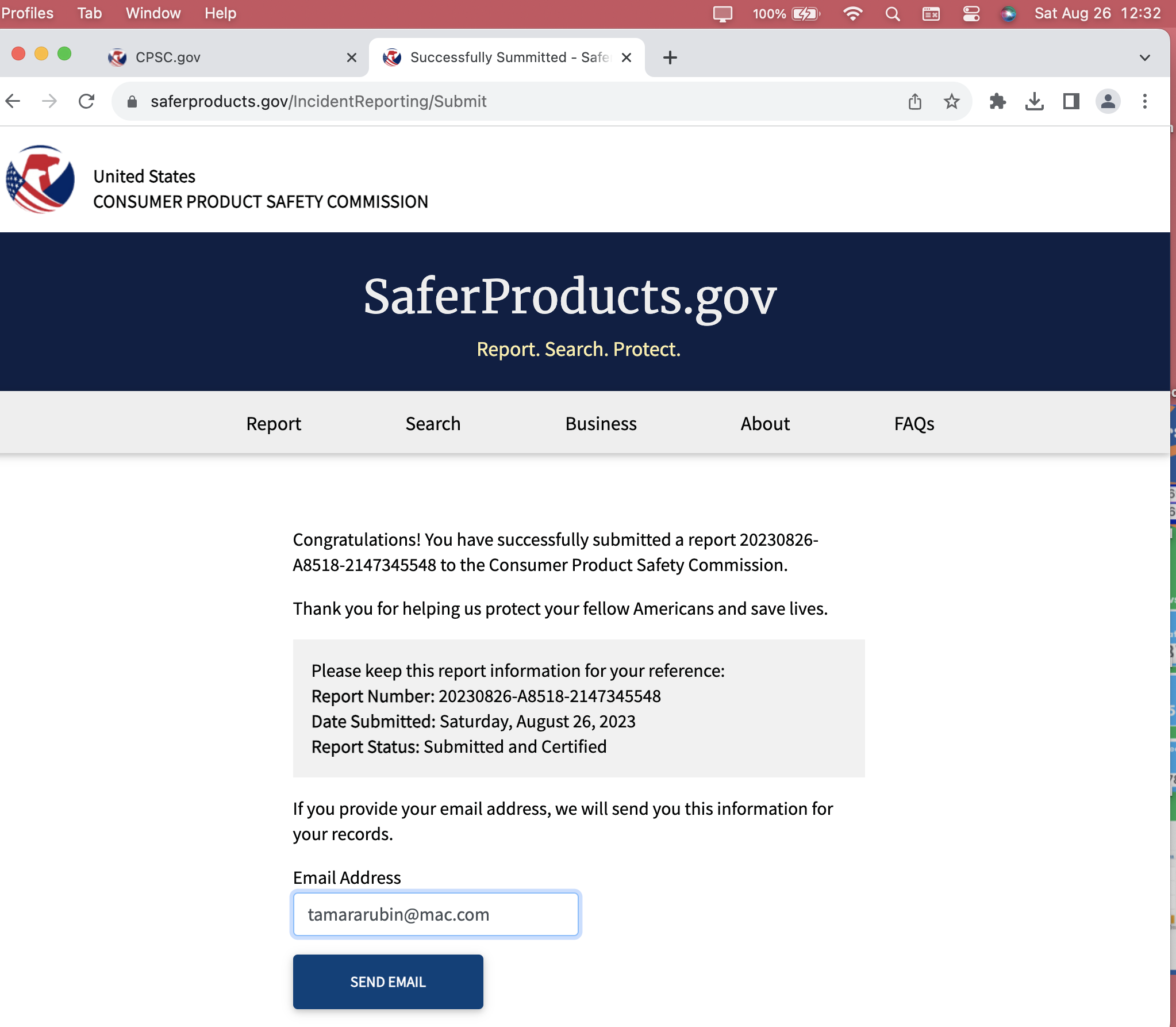
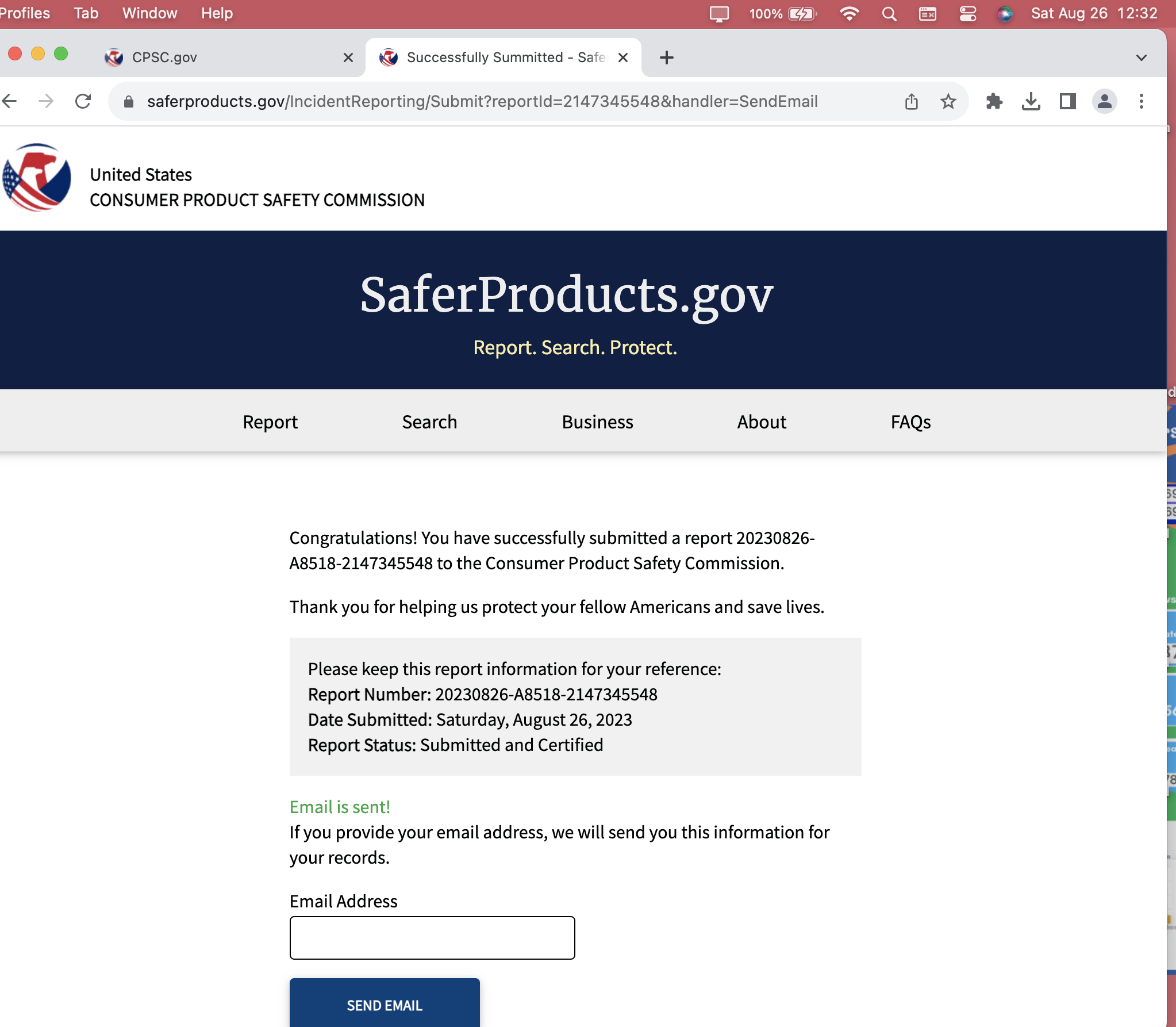
This is CPSC Incident Report Number:
20230826-A8518-2147345548
October 2023 Update
In October of 2023, less than two months after we published this report and submitted it to the CPSC (below) the legal team for Pura Stainless sent us a cease and desist letter asking us to take down this report and related articles. You can read that cease and desist letter as well as our responses on this link.
Violation Report Text, as Filed With the CPSC:
August 26, 2023 — Saturday
Summary/ Overview:
In 2017 and 2018 — and possibly later — Pura Stainless continued to sell high-Lead-content insulated stainless steel Pura Kiki baby bottles after learning of the following issue with their product: multiple documented instances of product failure resulting in child-accessible Lead at very high levels (as much as 800,000 ppm) in one component — far in excess of the CPSIA Lead-content ban for Lead in children’s products (limiting Lead in substrates to 100 ppm). Pura’s sales of Lead-contaminated children’s products on store shelves (virtual and brick and mortar) and distribution from warehouse inventory continued after the company stated (in writing to customers — see below) that they had begun to manufacture a Lead-free version of the product. Lead-contaminated versions of this product continued to be sold through at least 2018, and may have been sold as late as 2019 (possibly even later, until Lead-contaminated inventory was exhausted). These unsafe and illegal Lead-contaminated baby bottles are still in use in homes across the United States today in 2023, as no voluntary recall was ever announced by the manufacturer.
Separately, Made Safe (MadeSafe.org) knowingly continued to allow their certification to be used on the packaging of (and marketing for) these products – after learning that the products were in violation of the CPSIA due to unsafe levels of Lead which presented an exposure risk to the user (infants, toddlers and young children) with a common/frequent product failure due to the flawed design/ construction of the product. The Made Safe certification specifically certified these Lead-contaminated Pura children’s products as Lead-free (via language that ranged from stating that products with their certification were free of heavy metals/ free of toxic substances — including Lead) — a certification which was printed on the exterior paperboard packaging sleeve of Lead-contaminated versions of this product [likely until the Lead-contaminated inventory was exhausted].
We (the Lead Safe Mama, LLC parent advocacy community) request a full mandatory public recall announcement by the CPSC for this product (sold through at least 2018), along with an investigation to determine until which date the Lead-contaminated inventory continued to be manufactured and more specifically — until which date the Lead-contaminated version of this product continued to be sold. We also request that the CPSC make an example of both Made Safe and Pura Stainless — with criminal and civil fines and penalties levied to the extent allowable by law under the CPSIA for violating the CPSIA and not self-reporting the issue to the CPSC to coordinate a highly visible public recall.
Precedent for the CPSC following through on this recall (and related civil and criminal penalties for those involved) has been set with the CPSC recall of the Green Sprouts insulated stainless steel bottles in November of 2022 (essentially the same product, with the exact same issue/mode of failure) and with the Cupkin product recall in July 2023 (similar product with similar Lead-contaminated component); however, action in this case should include penalties directly proportionate to the intentional inaction (a de facto deliberate cover-up of the violation) by Pura Stainless and Made Safe, which was perpetrated over the course of years — and perpetrated in writing (by virtue of the false and misleading labeling on the packaging).
Violation Report
To our friends at the CPSC: Relevant to the CPSIA violation report detailed herewith, I wanted to first take a moment to thank you for the following three similar Lead-contaminated children’s product recalls – which (individually and collectively) constitute precedents supporting the need to implement an immediate recall for the product in question- the Lead-contaminated Pura Kiki insulated stainless steel baby bottles sold through at least 2018):
#1.) We first reported in detail on the CPSIA Lead-content violations with the Cupkin children’s cup products in January of 2023 (01/09/2023); the CPSC published an official recall notice for this product last week (7/20/2023). Thank you for this! This was just over a 6-month turnaround from the report of these findings to the recall (if you don’t count our initial/preliminary reporting of this violation on Social Media when we first discovered the issue in October of 2022.)
#2.) We first reported in detail on the CPSIA Lead-content violations with the Green Sprouts Insulated Stainless Steel Baby Bottles in September of 2022 (09/19/2022); the CPSC published an official recall notice for this product pretty quickly — just over two months later! (11/23/2022). [Your rapid turn-around for this particular recall was commendable!]
#3) We first reported that the Nuk glass baby bottles with gray and white stars were painted with high-Lead-paint on June 15, 2021; the CPSC officially announced that product recall more than one year later – last summer, on July 28, 2022 [Note: inexplicably/ troublingly, none of the other Lead-painted designs from that company were included in that recall — but hopefully it was a “first step???”]
I am writing to you today on behalf of the Lead Safe Mama, LLC parent-advocacy community about a CPSIA Lead Content violation that I last reported directly to you in July of 2018 (five years ago) — and for which I had, frankly, basically given up on continuing to expect any action from the CPSC. After receiving no response or action to multiple communications (direct e-mails exchanges with your officers and employees (in 2018) and later), 3-1/2 years later (in December of 2021) we sent an additional e-mail report comprehensively detailing the violations, with specific products and XRF test results for each product, through a “back channel” (a personal friend who is a former high-ranking government official, and who knows some folks at the CPSC) — for which we also received no response <crickets>.
The three Lead Safe Mama, LLC precipitated official CPSC recalls noted above support the argument that this aforementioned product which I last communicated with you directly about in 2018 (and then followed up with you about through unofficial channels in December of 2021) needs your attention and needs it now before more harm can be done to children.
I will start by reiterating what I have now shared many times before. This particular bottle (and the manufacturing failure and use of illegal amounts of Lead in one component) was determined to be the likely primary source of exposure for one child who was Lead-poisoned [the child lived in a newer construction home, and extensive due diligence had already been carried out, without being able to find any additional sources of possible significant Lead exposure. Details on this are below. [Note: This is not an isolated incident; there are additional similar cases.]
The product also has the exact same design/construction issue as recall #2 noted above – the Green Sprouts stainless bottle – which is also a similar issue/concern for which the Cupkin products were finally officially recalled last month. Here are the details about the product that should be recalled, just as the products noted above were recalled:
- ONE) Product Name: Pura Brand — Pura Kiki model — insulated stainless steel baby bottle [this issue does not pertain to the non-insulated version of this bottle].
- TWO) Dates Manufactured: All years prior to and including through mid-2018, and possibly later dates (a date through which the product continued to be manufactured with high levels of Lead would need to be determined via a CPSC audit of the manufacturing processes of the manufacturer).
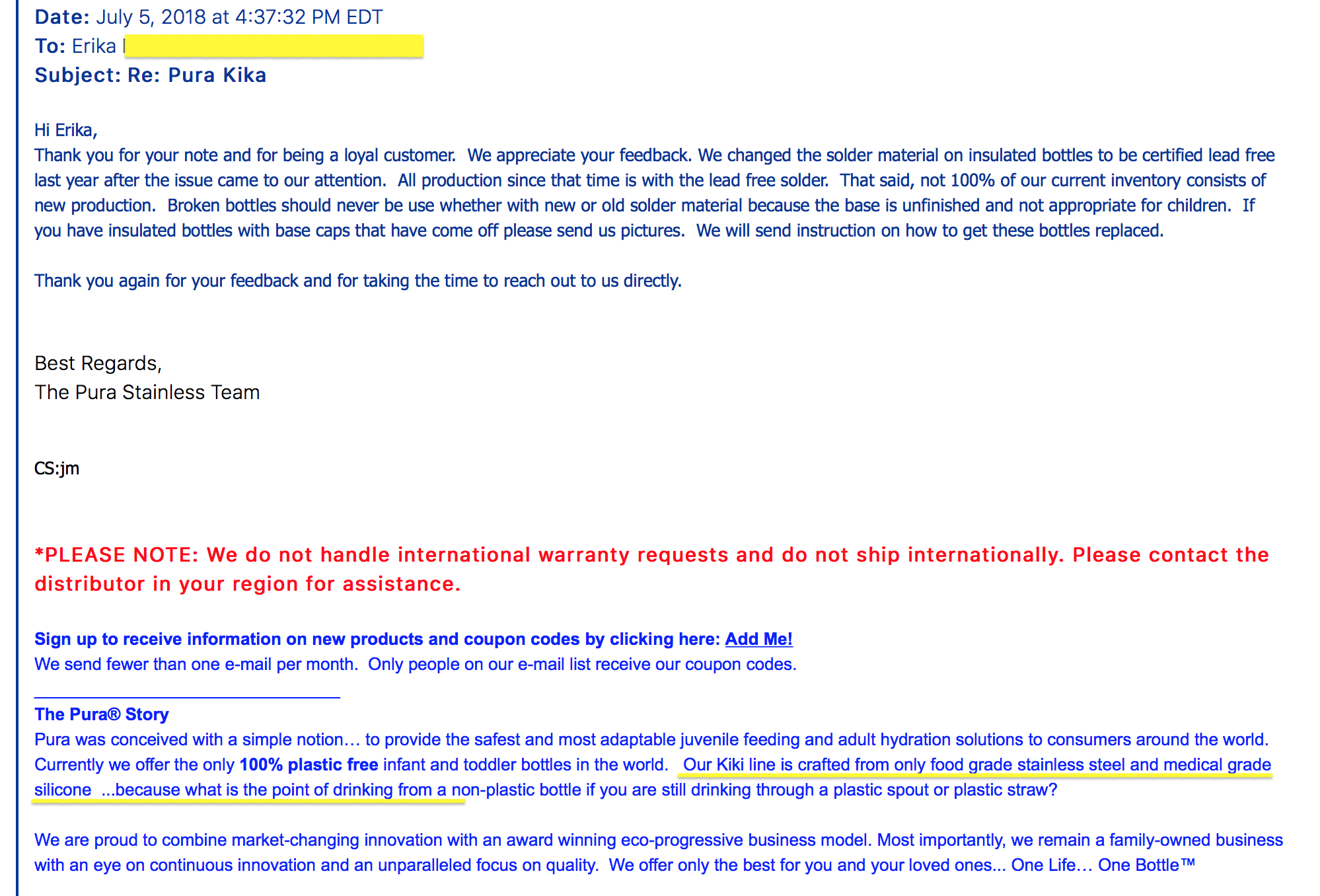
- THREE) Dates Sold: As no recall was done at the time we first made the manufacturer aware of this concern in 2016/2017 (the product was not pulled from store shelves, nor was it pulled from distribution centers — as clearly indicated from the communications from the manufacturer, images attached), and as our inquires determined that the company’s statements explicitly indicated that they were continuing to distribute and sell the Lead-contaminated product until the Lead-contaminated inventory was exhausted — with our sources indicating likely tens of thousands of units warehoused at that time, it is possible that units of the Lead-contaminated (illegal and unsafe) version of the insulated stainless steel Pura Kiki baby bottles was sold online and through retail outlets through 2019 and possibly later. We have published correspondence from Pura Kiki dated July 7, 2018, where they admit that they are continuing to sell their (older) Lead-contaminated stock (see website) despite having been directly made aware of the Lead issue in 2017.
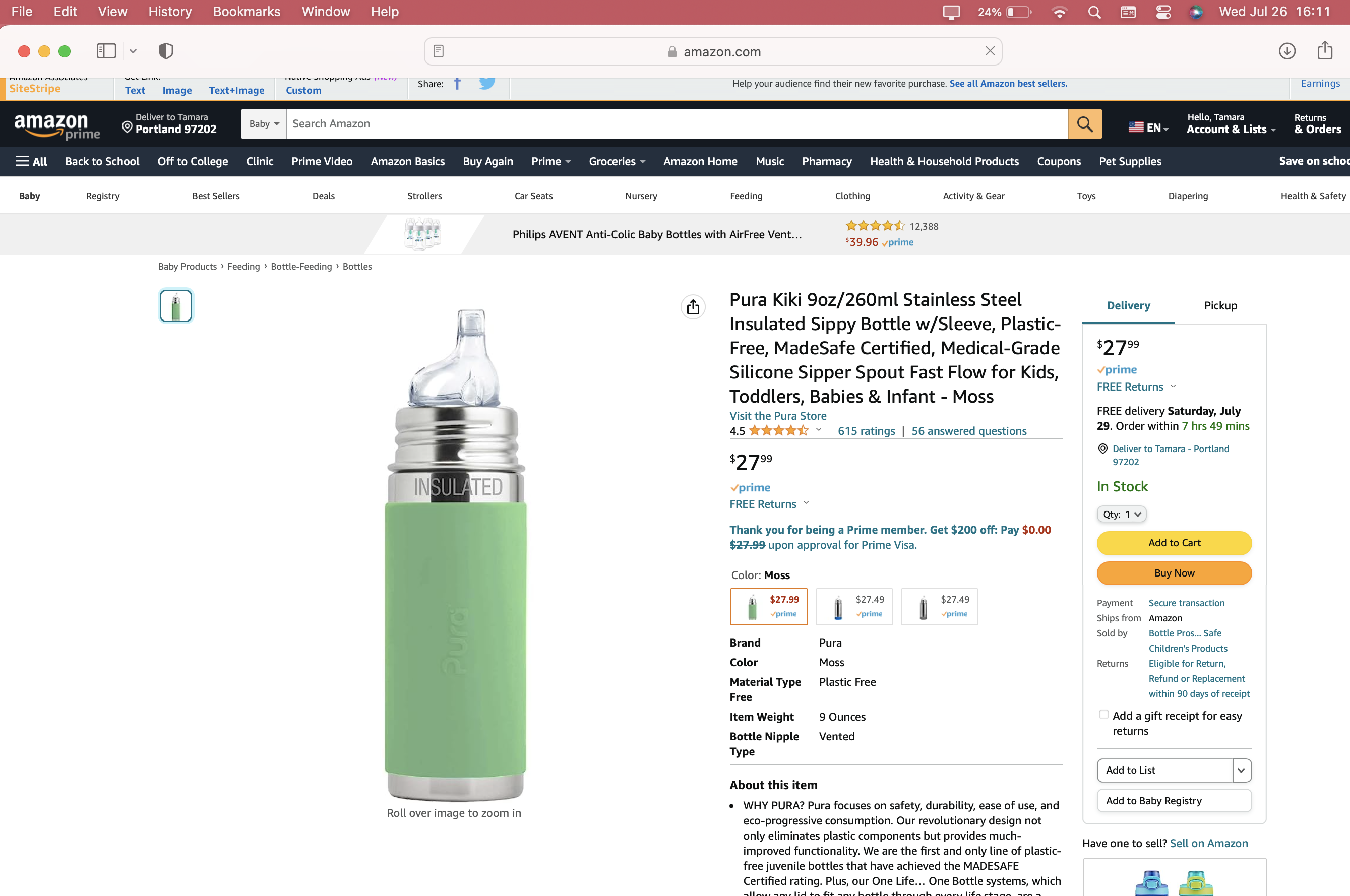
- FOUR) Compounding Factor: Product Durability/ Intended Duration of Use: A primary compounding factor with this product is that it was marketed and sold as an item that can be used throughout a child’s entire early childhood. As a result, if a parent bought this Lead-contaminated bottle to use for their newborn in 2018 or 2019 (or even in earlier years) the intention — by design, and in confirmed practice) was that the child would still be using the product continuously when they were four or five or six years old. We have found this to be the case — see point number nine below. Their slogan, “Baby Grows…Bottle Evolves” underscores this concern [see attached image with their marketing language relevant to this product]. In support of any investigation you may undertake in this matter, we have a full timeline indicating the company’s knowledge of the issue — and the fact that they continued to sell Lead-contaminated bottles, in spite of this knowledge — on our website (LeadSafeMama.com).
- FIVE) Compounding Factor: Fraudulent/ Misleading Packaging with Made Safe Certification Language: The company used and continues to use language including the following phrases (which would indicate to the consumer that the product is Lead-free): “Made Safe Certified”; “Eco-progressive”; “Toxin-Free”; “Kids Safety and Sustainability First”…
- SIX) Date Issue First Reported by Lead Safe Mama, LLC: April/ May 2017
- SEVEN) Dates of Documented (saved) Communication Thread With CPSC About This Product: We have several saved communications with the CPSC about this product specifically and this issue in general from July 2018 (see our website for full communication thread). There were also earlier communications about this issue but we did not save copies. After this exchange from July of 2018, we never received a response or determination of the next steps. These communications were with the following parties: Stephanee Synnott Ph.D., Jonathan Midgett Ph.D., and DeWane Ray. We were told on July 30, 2018, that Dr. John Boja would get back to us. We never heard back from anyone after this communication about this issue.
- EIGHT) Date of Follow-Up Letter to CPSC (sent to CPSC through a former CPSC official as we had not received any response to our previous communications): December 2021 (see our website for full communication).
- NINE) 2023 Issue With This Product: Lead Safe Mama, LLC is a small business that does in-home visits with families of Lead-poisoned children around the country. This year (in the first 7 months of 2023) we have worked with several families who had a confirmed Lead-contaminated version of this product still in use in their home. There have been two common scenarios: #1) a child who had the bottle as an infant is now using the bottle at 4, 5, or 6 years old; and #2) the family has had a second or third child since they purchased this bottle 4 or 5 or 6 years ago, and they are now using the bottle (again) for their youngest infant. There is also a concern for these bottles (due to the promised durability of the product) to be handed down from one family to another. Each of these scenarios creates the opportunity for these bottles to poison children for years into the future, which is why we are demanding an investigation and recall of these bottles now, even though the company states that the current version of their products no longer uses Lead to create their vacuum seal [they claim this is true for versions manufactured since mid-2018 (in a written response when pressed on the subject by a customer who also happened to be a reader of our posts, and forwarded that communication to us)].
Detailed Description of Incident With Child Poisoned by This Product:
The CPSIA violation represented by this specific product was brought to our attention when we worked with a child (with a family in Portland, Oregon) with a “mysterious” (stubbornly unidentifiable) source of Lead-exposure. Upon working with the family and conducting an exhaustive evaluation of the home and the contents of the home, we determined that this bottle was the likely primary (and possibly sole) source of Lead exposure for this child… The bottom cap had fallen off of the bottle, but the mother had not realized there was any issue (she assumed the product was safe), and continued to let her infant use the bottle. The child would use the bottle at mealtime and had developed an (“age-appropriate”) habit of smashing his oatmeal with the bottom of his bottle. The bare Lead sealing dot (tested with an XRF instrument and determined to be positive for at least 800,000 ppm Lead) was coming into direct contact with his daily porridge as a result of this habit – the almost certain cause of the “mysterious” elevated blood Lead level in this child. Fast forward 6 years later and this child is – predictably – now struggling with learning disabilities and other behavioral concerns related to his Lead exposure as an infant. The illegal (extremely high) Lead content of these bottles, along with the well-documented propensity for the bottle’s bottom cap to fail and pop off present a very real Lead-exposure/ poisoning risk to the children using these bottles.
FAILURE OF COMPLIANCE WITH DUTY TO REPORT:
To our knowledge, since the time the company (Pura Stainless) was made aware of this issue (the illegal levels of Lead in the sealing dot of their Pura Kiki stainless baby bottles), Pura has not made any public statements to customers about the issue, nor have they issued a voluntary recall for the product nor did they self-report the violation to the CPSC that we are aware of (as is required by law when a company learns of a CPSIA violation in a product they manufacture that is intended for use by children).
We are referring to the self-reporting requirement noted on this CPSC link, https://www.cpsc.gov/Business–Manufacturing/Recall-Guidance, under the “Duty To Report” tab:
“If you are a manufacturer, importer, distributor, and/or retailer of consumer products, you have a legal obligation to immediately report the following types of information to the CPSC:
-
-
- A defective product that could create a substantial risk of injury to consumers;
- A product that creates an unreasonable risk of serious injury or death;
- A product that fails to comply with an applicable consumer product safety rule or with any other rule, regulation, standard, or ban under the CPSA or any other statute enforced by the CPSC;
- An incident in which a child (regardless of age) chokes on a marble, small ball, latex balloon, or other small part contained in a toy or game and that, as a result of the incident, the child dies, suffers a serious injury, ceases breathing for any length of time, or is treated by a medical professional; and
- Certain types of lawsuits. (This applies to manufacturers and importers only and is subject to the time periods detailed in Sec. 37 of the CPSA).
-
Failure to fully and immediately report this information may lead to substantial civil or criminal penalties. CPSC staff’s advice is “when in doubt, report.”
Especially given the egregious actions of this company (the fact that they knowingly continued to sell their contaminated products until the inventory ran out) we hope you use this measure — the allowance for civil and criminal penalties in this matter, to the fullest extent possible — to discourage Pura and other baby bottle manufacturers from manufacturing any children’s products with high levels of Lead in any component.
SEPARATE BUT EQUAL CONCERN — DECEPTIVE MARKETING:
These stainless steel insulated Lead-contaminated Pura Kiki baby bottles were knowingly sold by Pura with prominent “Made Safe Certified” packaging — a certification that claims to assure customers a product is Lead-free. Upon being made aware of the issue (the presence of Lead in Made Safe’s certified Lead-free bottles), the Founder and Executive Director of the Made Safe organization specifically, knowingly, and intentionally refused to remove the Made Safe certification from the product, and also refused to demand that manufacturer stop using the packaging with the Made Safe certification. In this case, there is clearly a dual culpability in selling Lead-contaminated products for use by infants, and we request that you investigate Made Safe‘s role in this deception — and consider also fining them for their failure of compliance with a duty to report in this matter (although I understand holding a certification agency responsible for a duty to report in this case may be unprecedented). We are including six images with this report, including screenshots of communications. All of these images (and this report with proper formatting) can be found on our website at the following link: https://tamararubin.com/2023/08/cpsc-violation-report-august-2023-pura-stainless-knowingly-sold-lead-contaminated-childrens-products-through-at-least-2018-there-was-no-recall-these-products-are-still-in-use-in-homes-today/.
Images of failed product
Communications from Pura
January 17, 2018
July 5, 2018
Amazon Images & Marketing From July 2023
Confirmations from this August 26, 2023 — Submission
Report #: 20230826-A8518-2147345548
Never Miss an Important Article Again!
Join our Email List














Does this lead stuff have anything to do with their regular bottles (uninsultated ones):
https://www.amazon.com/gp/product/B019JBA730/ref=ppx_yo_dt_b_search_asin_image?ie=UTF8&psc=1
I am wondering because we used these exclusively when my daughter was a baby for years and she now has some neurodivergent issues – wondering if it is connected?