#AskTamara: Interesting note, melting point of lead…
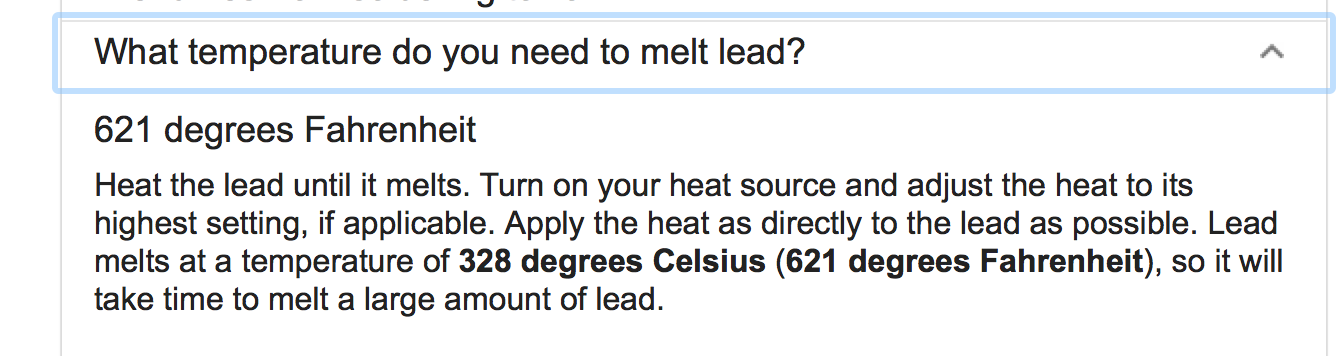
- What is the melting point of lead?
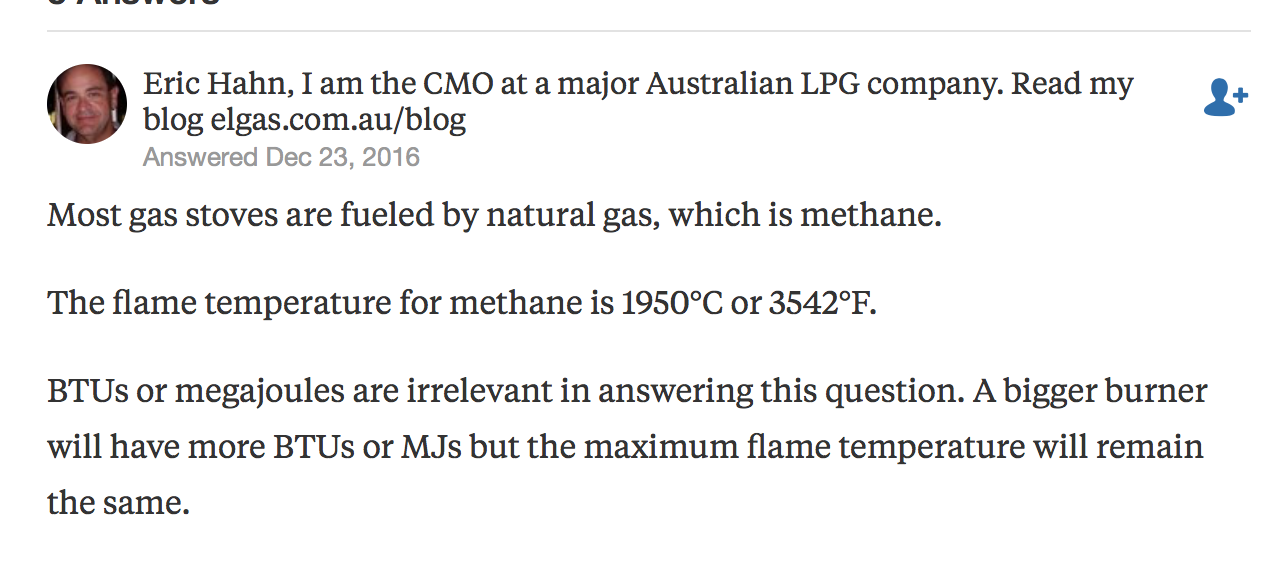
- How hot does the flame on a gas stove get.
More questions came up after my initial post, so these have been added below too, with their answers.
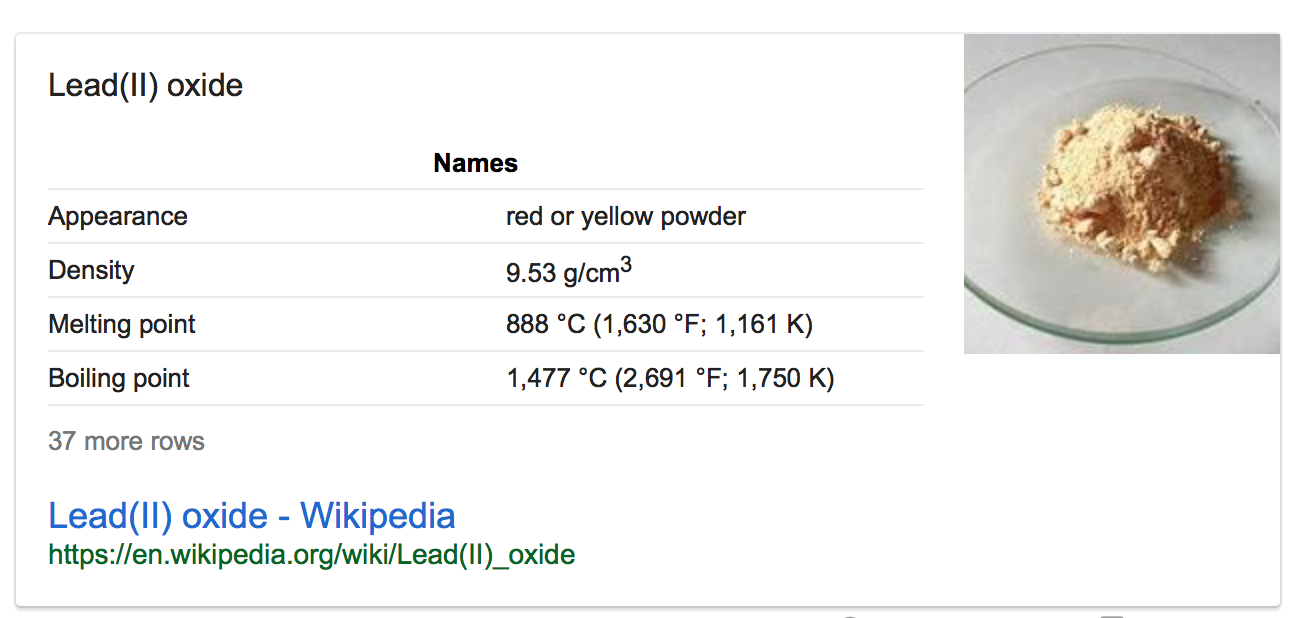
- What’s the melting point of lead oxide?
- What is lead oxide used for?
This inquiry is especially interesting to me, as my children were poisoned by inhaling “lead fumes”. I have, however, learned (over the years) that calling it “lead fumes” is a bit of a misnomer. It is not like solid lead was melted and inhaled, nor like solid lead oxide was melted and inhaled. The lead paint was melted off the house (lead oxide-containing paint) using an open flame torch.
Elemental lead (or lead oxide) itself does not have to be melted to create the fumes that can poison a child. The “fuming” is created by the heating and deterioration of the many components that are present in the coating alongside the micro-particulate lead pigment base. In the example of my children, the paint (which in that type of housepaint is typically linseed oil and other elements in emulsion along with the lead particles) was heated to the point where it created fumes containing lead micro-particulates… not necessarily “lead fumes”. These fumes containing lead particulates were then inhaled by my children.
To Note: Even “heat guns” [vs. heating by open–flame torch, as was the case with my children] heating lead-containing paint to “just” 700 degrees (and even to temperatures below this) can “fume” the lead oxide to the degree that it can poison a child. [Again, the issue is semantics… around the idiomatic usage of the term “lead fumes”to refer to micro-particulate lead suspended in smoke or fumes, which is well below the temperature required for the lead itself to actually melt/vaporize.]
Does that makes sense? Let me know you if have questions!
First and foremost (in the question of cookware) I believe we need to be governed by the principle found in Hippocrates’ (“Father of Medicine) famous medical oath of “First, Do No Harm”, and until it can be proven that high-lead-containing exterior decorative elements on cookware (see this link, this link and this link) are safe when repeatedly exposed to high temperatures over their life span (a gas stove top’s high-temperature flames – see below), lead in cookware should be avoided entirely.
These are the answers I found to the above questions:
Melting point of lead:
Melting point of lead oxide:
Also curious (“What is lead oxide used for?”)
How hot do flames on a gas stove get:
Never Miss an Important Article Again!
Join our Email List